Public Advisories
Apotex is notifying all direct account Retailers, Wholesalers and Distributors that have received an impacted lot of a voluntary recall being conducted to the Retail Level for selected lots of APO-K (Potassium Chloride Slow-Release Tablets) distributed in some International markets.
Additional Lots Added: Voluntary Nationwide Recall of 21 Lots of Piperacillin and Tazobactam For Injection, USP 40.5 Grams Due to Possibility of Precipitation / Crystallization In IV Bag Or IV Line Upon Reconstitution
Apotex Corp. announced today that it is conducting, on behalf of the manufacturer Hospira, Inc., a voluntary nationwide recall of 21 lots of Piperacillin and Tazobactam for Injection, USP 40.5 grams, NDC number 60505-0773-00 to the hospital / healthcare provider / user level. The impacted lots of Piperacillin and Tazobactam for Injection, USP 40.5 grams may show precipitation / crystallization in IV bag or IV line after reconstitution.
Hospira has stated that administration of precipitated Piperacillin / Tazobactam in an IV bag or IV line may result in local reactions such as phlebitis, renal impairment, end-organ embolism and ischemia, and/or vasculitis (because the precipitate was visible, its particles may be large enough to cause these adverse events). In addition, the precipitation of the drug may not allow delivering a needed therapeutic dose of piperacillin and tazobactam, thus resulting in inadequate treatment of the targeted infection. This could result in adverse health consequences that could range from transient and minor impairment or complaints to permanent impairment of a body function or permanent damage to a body structure. Hospira has not received any reports of adverse events related to this recall.
The product is indicated for the treatment of patients with moderate to severe infections caused by piperacillin-resistant, piperacillin/tazobactam-susceptable, β-lactamase producing strains of the designated microorganisms in the specified conditions such as, Appendicitis, Uncomplicated and Complicated skin and skin structures infections, Postpartum Endometritis or Pelvic Inflammatory disease, Community Acquired Pneumonia and Nosocomial Pneumonia. For further details, please refer to the package insert. The product is packaged in 300 mL glass vials for reconstitution. The affected Piperacillin and Tazobactam for Injection, USP 40.5 grams lots include the following:
| Lot Number | Expiry Date |
|---|---|
| 503B023 | 08/2013 |
| 503B028 | 11/2013 |
| 503B029 | 11/2013 |
| 503B030 | 11/2013 |
| 503B031 | 11/2013 |
| 503B032 | 11/2013 |
| 503B033 | 11/2013 |
| 503C001 | 12/2013 |
| 503C002 | 12/2013 |
| 503C003 | 12/2013 |
| 503C004 | 12/2013 |
| 503C009 | 01/2014 |
| 503C010 | 02/2014 |
| 503C011 | 02/2014 |
| 503C012 | 02/2014 |
| 503C014 | 02/2014 |
| 503C015 | 04/2014 |
| 503C016 | 04/2014 |
| 503C017 | 04/2014 |
| 503C019 | 04/2014 |
| 503C020 | 04/2014 |
The product can also be identified by NDC number 60505-0773-00 and UPC 360505077304. The product was distributed nationwide in the United States to wholesalers, distributors, HMOs, home infusion and long term care service providers.
The product and all recalled lots are manufactured by Hospira. Apotex is the U.S. distributor of the product and is conducting the recall at the request and on behalf of Hospira. Hospira is investigating to determine the root cause.
Apotex will notify its direct account customers by sending the recall notification letter by UPS 2nd day air service and is arranging for product return of all recalled product.
Anyone with an existing inventory of the product should stop use and distribution, quarantine the recalled lots immediately and call GENCO at 1-877-674-2078 between the hours of 7 a.m. to 5 p.m. CST, Monday through Friday, to arrange for their return.
For clinical inquiries, please contact Hospira using the information provided below:
| Hospira Contact | Contact Information | Areas of Support |
|---|---|---|
| Hospira Global Complaint Management | 1-800-441-4100 (8am-5pm CT, M-F) (ProductComplaintsPP@hospira.com) | To report adverse events or product complaints |
| Hospira Medical Communications | 1-800-615-0187 or medcom@hospira.com (Available 24 hours a day/7 days per week) | Medical inquiries |
Any adverse reactions or quality problems experienced with the use of this product may be reported to the U.S. Food and Drug Administration's (FDA) MedWatch Adverse Events Program either online, by regular mail or by fax.
- Online: www.fda.gov/medwatch/report.htm
- Regular mail: use postage-paid, pre-addressed Form FDA3500 available at www.fda.gov/MedWatch/getforms.htm
- Fax: 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration (FDA).
Apotex Inc.
For further information:
Steve Giuli
Director of Government Affairs and Industry Relations
Apotex Corp.
Direct: 301-654-4964
sgiuli@apotex.com
Voluntary Nationwide Recall of 15 Lots of Piperacillin and Tazobactam for Injection, USP 40.5 Grams due to Possibility of Precipitation / Crystallization in IV Bag or IV Line Upon Reconstitution
Apotex Corp. announced today that it is conducting, on behalf of the manufacturer Hospira, Inc., a voluntary nationwide recall of 15 lots of Piperacillin and Tazobactam for Injection, USP 40.5 grams, NDC number 60505-0773-00 to the hospital / healthcare provider /user level. The impacted lots of Piperacillin and Tazobactam for Injection, USP 40.5 grams may show precipitation / crystallization in IV bag or IV line after reconstitution.
Hospira has stated that administration of precipitated Piperacillin / Tazobactam in an IV bag or IV line may result in local reactions such as phlebitis, renal impairment, end-organ embolism and ischemia, and/or vasculitis (because the precipitate was visible, its particles may be large enough to cause these adverse events). In addition, the precipitation of the drug may not allow delivering a needed therapeutic dose of piperacillin and tazobactam, thus resulting in inadequate treatment of the targeted infection. This could result in adverse health consequences that could range from transient and minor impairment or complaints to permanent impairment of a body function or permanent damage to a body structure. Hospira has not received any reports of adverse events related to this recall.
The product is indicated for the treatment of patients with moderate to severe infections caused by piperacillin-resistant, piperacillin/tazobactam-susceptable, β-lactamase producing strains of the designated microorganisms in the specified conditions such as, Appendicitis, Uncomplicated and Complicated skin and skin structures infections, Postpartum Endometritis or Pelvic Inflammatory disease, Community Acquired Pneumonia and Nosocomial Pneumonia. For further details, please refer to the package insert. The product is packaged in 300 mL glass vials for reconstitution. The affected Piperacillin and Tazobactam for Injection, USP 40.5 grams lots include the following:
| Lot Number | Expiry Date |
|---|---|
| 503B015 | 04/2013 |
| 503B016 | 04/2013 |
| 503B019 | 07/2013 |
| 503B020 | 07/2013 |
| 503B021 | 07/2013 |
| 503B022 | 07/2013 |
| 503B024 | 08/2013 |
| 503B025 | 09/2013 |
| 503B026 | 09/2013 |
| 503B027 | 09/2013 |
| 503C005 | 12/2013 |
| 503C006 | 12/2013 |
| 503C007 | 01/2014 |
| 503C008 | 01/2014 |
| 503C018 | 04/2014 |
The product can also be identified by NDC number 60505-0773-00 and UPC 360505077304. The product was distributed nationwide in the United States to wholesalers, distributors, HMOs, home infusion and long term care service providers.
The product and all recalled lots are manufactured by Hospira. Apotex is the U.S. distributor of the product and is conducting the recall at the request and on behalf of Hospira. Hospira is investigating to determine the root cause.
Apotex will notify its direct account customers by sending the recall notification letter by UPS 2nd day air service and is arranging for product return of all recalled product.
Anyone with an existing inventory of the product should stop use and distribution, quarantine the recalled lots immediately and call GENCO at 1-877-674-2078 between the hours of 7 a.m. to 5 p.m. CST, Monday through Friday, to arrange for their return.
For clinical inquiries, please contact Hospira using the information provided below:
| Hospira Contact | Contact Information | Areas of Support |
|---|---|---|
| Hospira Global Complaint Management | 1-800-441-4100 (8am-5pm CT, M-F) (ProductComplaintsPP@hospira.com) | To report adverse events or product complaints |
| Hospira Medical Communications | 1-800-615-0187 or medcom@hospira.com (Available 24 hours a day/7 days per week) | Medical inquiries |
Any adverse reactions or quality problems experienced with the use of this product may be reported to the U.S. Food and Drug Administration's (FDA) MedWatch Adverse Events Program either online, by regular mail or by fax.
- Online: www.fda.gov/medwatch/report.htm
- Regular mail: use postage-paid, pre-addressed Form FDA3500 available at www.fda.gov/MedWatch/getforms.htm
- Fax: 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration (FDA).
Apotex Inc.
For further information:
Steve Giuli
Director of Government Affairs and Industry Relations
Apotex Corp.
Direct: 301-654-4964
sgiuli@apotex.com
On April 9, 2013, Apotex issued a public advisory regarding a Type I & II voluntary recall of one lot of ALYSENA 28 (levonorgestrel and ethinyl estradiol tablets USP) birth control pills – specifically, lot number LF01899A – due to a packaging flaw. In parallel with this recall, Apotex has been working closely with the third party fabricator, Laboratorios Leon Farma, S.A., to ensure all necessary quality controls for this product are effective.
To date, there is no evidence that the packaging flaw with this one lot of ALYSENA 28, which involved an incorrect placement and number of active tablets (pink) and placebo tablets (white) in the blister, is repeated in any other lots of ALYSENA 28. However, as a precautionary measure while Apotex investigates why the original lot of ALYSENA 28 contained blisters with extra placebo tablets (white) in place of active tablets (pink), Apotex is now expanding the scope of the voluntary Type I & II recall to all lots of ALYSENA 28 distributed to date, having the following lot numbers:
LF01901A, LF01900A, LF01898A, LF01894B, LF01980A, LF01982A, LF01981A, LF01979A, LF02037A, LF02036A, LF02026A
Furthermore, Apotex is undertaking a full visual inspection of each blister from all new lots of ALYSENA 28 that Apotex has received from the third party fabricator, to ensure there are no other packaging flaws or other defects before these lots are distributed in Canada.
Apotex is proactively taking these additional measures to ensure that this product meets Apotex's high quality standards.
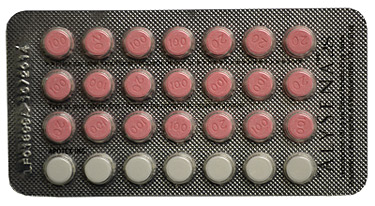
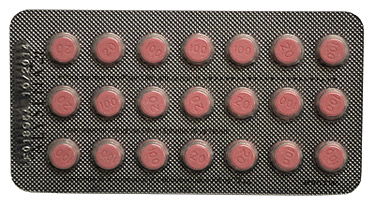
ALYSENA 21, which is manufactured by the same third party fabricator at the same facility, is not part of the current recall, as the blisters for this product do not normally include a row of placebo tablets (white). However, because this product is made in the same facility as ALYSENA 28, Health Canada and Apotex are working together to verify this product as well. Individuals are encouraged to check their blisters of ALYSENA 21 to confirm that they contain three rows of seven active tablets (pink), for a total of 21 pink tablets.
According to the product monograph for ALYSENA 28, in the case of a missed dose, individuals should use a non-hormonal method of contraception as an interim measure until they speak with their pharmacist and/or physician and obtain medical advice.
For sexually active individuals, missing a week of active tablets (pink) would place an individual at the same risk level of becoming pregnant as an individual who is not using contraception.
Apotex is asking any individuals who have received any of the identified lots of ALYSENA 28 to speak with their pharmacist and/or physician and return the blister packets to their pharmacy. Apotex has also established a toll-free recall information service at 1-866-367-4537 to provide patients and health care professionals with additional support.
Correct ALYSENA 28 blister pack configuration
Correct ALYSENA 21 blister pack configuration
Apotex had initiated a voluntary Type II recall to the retail and pharmacy level for Alysena™ 28 (lot LF01899A), on behalf of the fabricator, Laboratorios Leon Farma S.A., due to the potential for packaged unit to contain two rows of placebo instead of one row (14 tablets instead of 7 tablets) and two rows of active contraceptive tablets (14 tablets instead of 21 tablets) in the blister pack.
Apotex has been swift to respond to Health Canada's additional classification of Type I assigned to a proportion of the patient population associated with patients who should not get pregnant, whether for medical reasons or exposure to agents detrimental to a developing fetus (such as those on pregnancy prevention programmes while taking drugs that can cause harm to a developing fetus).
As such, and according to the product monograph, in the case of a missed dose, use a non-hormonal method of contraception as an interim measure until you speak with your pharmacist / physician and obtain medical advice. If you have received this lot (LF01899A) we are asking those patients to speak with their pharmacist and or physician and return any units of this lot to their pharmacist.
Correct AlysenaTM28 blister pack configuration
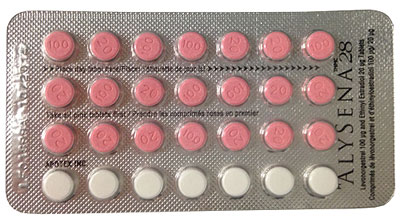
Rows 1, 2 and 3: Seven (7) pink tablets per row (21 active pills) ; Row 4: Seven (7) white tablets (inactive/placebo pills).
Any other configuration is incorrect, and should be returned to pharmacy.
Apotex is notifying all direct account Wholesalers, Distributors, and Retailers that have received impacted lots of a voluntary CLASS II recall being conducted up to the Retail level for Piperacillin and Tazobactam for Injection, USP. This voluntary CLASS II recall is being conducted in coordination with the US Food and Drug Administration.




