*All other brand or product names located in this site are Trademarks of their respective holders.
Guanfacine Extended-Release Tablets
NDC Number :
60505-3927-01
Material Number :
68365
Active Ingredient :
Guanfacine
Bioequivalent to * :
INTUNIV®
Therapeutic Class (AHFS) :
MISCELLANEOUS CENTRAL NERVOUS SYSTEM AGENTS
Strength :
1MG
Pack Size (Form) :
100 TABLET (BOTTLE)
Dosage Form :
Tablet
Scored :
NO
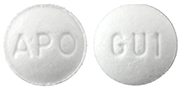
Color :
White to off-white
Shape :
Round, biconvex
Markings :
Engraved "APO" on one side, "GU1" on the other side
Route of Administration :
Oral
Storage Condition :
Controlled Room Temperature (68 - 77 degrees F)
Minimum Order Quantity :
12
Multiple Order Quantity :
12
Latex Free :
Yes
Sugar Free :
Yes
Dye Free :
Yes
Alcohol Free :
Yes
Preservative Free :
Yes
Hazardous Material :
No
Product Resources :
Prescribing Information (913 KB)
Patient Information Leaflet (493 KB)

not to scale
Packaging Information :
| Length | Width | Height | Weight | Units/Pack | |
|---|---|---|---|---|---|
| Unit | 1.8 in | 1.8 in | 3.1 in | 0.07 lb | N/A |
| Inner | 7.1 in | 5.3 in | 3.1 in | 0.88 lb | 12 |
| Case | 16.2 in | 7.1 in | 7.4 in | 4.93 lb | 60 |
 United
States |
United
States | 


